

PRINCETON, NJ, USA I AugI Bristol Myers Squibb (NYSE: BMY) today announced the Phase 3 CheckMate -577 trial evaluating Opdivo (nivolumab) as an adjuvant therapy for patients with resected esophageal or gastroesophageal junction (GEJ) cancer met its primary endpoint of disease-free survival (DFS) at a pre-specified interim analysis. Second tumor, in addition to melanoma, where Opdivo has demonstrated a benefit in the adjuvant setting (ASCO®) and does not necessarily reflect the ideas and opinions of ASCO®.Opdivo is the first and only treatment to demonstrate superior efficacy in patients with esophageal or gastroesophageal junction cancer following neoadjuvant chemoradiation therapy and resection The content in this post has not been reviewed by the American Society of Clinical Oncology, Inc.
#CHECKMATE 577 FULL#
For full disclosures of the study authors, visit. The investigators concluded, “Among patients with resected esophageal or gastroesophageal junction cancer who had received neoadjuvant chemoradiotherapy, disease-free survival was significantly longer among those who received nivolumab adjuvant therapy than among those who received placebo.”ĭisclosure: The study was funded by Bristol Myers Squibb and Ono Pharmaceutical. The most common adverse events of any grade considered related to treatment were fatigue (17%), diarrhea (17%), pruritus (10%), and rash (10%) in the nivolumab group, and diarrhea (15%) and fatigue (11%) in the placebo group. Among adverse events considered related to study treatment, grade 3 or 4 adverse events occurred in 13% vs 6% of patients, serious adverse events occurred in 8% vs 3%, and adverse events led to discontinuation of treatment in 9% vs 3%. Treatment was discontinued due to adverse events of any cause in 13% vs 8%.
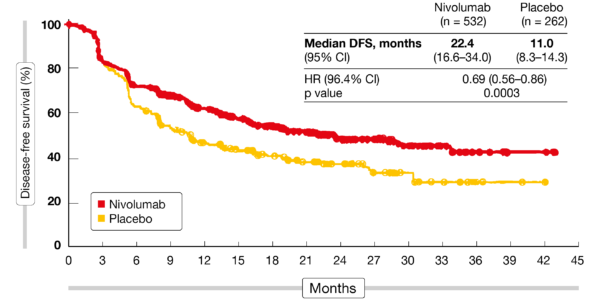
Serious adverse events of any cause occurred in 30% of patients in each group. Grade 3 or 4 adverse events of any cause occurred in 34% of patients in the nivolumab group vs 32% in the placebo group. Nivolumab benefit was observed irrespective of PD-L1 expression status or histology.Adjuvant nivolumab significantly prolonged disease-free survival vs placebo.Median distant metastasis–free survival was 28.3 months (95% CI = 21.3 months–not estimable) in the nivolumab group vs 17.6 months (95% CI = 12.5–25.4 months) in the placebo group (HR = 0.74, 95% CI = 0.60–0.92).

In a post hoc analysis, nivolumab was associated with benefit among 376 vs 187 patients with adenocarcinoma (median = 19.4 vs 11.1 months, HR = 0.75, 95% CI = 0.59–0.96) and among 155 vs 75 patients with squamous cell carcinoma (median = 29.7 vs 11.0 months, HR = 0.61, 95% CI = 0.42–0.88).ĭistant recurrence occurred in 29% vs 39% of patients and locoregional recurrence in 12% vs 17%. 001).ĭisease-free survival favored nivolumab across all prespecified subgroups examined, including among 89 vs 40 patients with known tumor cell PD-L1 expression ≥ 1% (median = 19.7 vs 14.1 months, HR = 0.75, 95% CI = 0.45–1.24) and among 374 vs 196 patients with known expression < 1% (median = 21.3 vs 11.1 months, HR = 0.73, 95% CI = 0.57–0.92). Median disease-free survival was 22.4 months (95% confidence interval = 16.6–34.0 months) in the nivolumab group vs 11.0 months (95% CI = 8.3–14.3 months) in the placebo group (hazard ratio = 0.69, 96.4% CI = 0.56–0.86, P <. The primary endpoint was disease-free survival.Īt the time of interim analysis (clinical data cutoff in May 2020), median follow-up was 24.4 months (range = 6.2–44.9 months). The maximum duration of the trial intervention period was 1 year. They were randomly assigned 2:1 between July 2016 and August 2019 to receive nivolumab at 240 mg every 2 weeks for 16 weeks followed by 480 mg every 4 weeks (n = 532) or placebo (n = 262).

The double-blind trial included 794 patients from sites in 29 countries with resected (R0) stage II or III esophageal or gastroesophageal junction cancer who had received neoadjuvant chemoradiotherapy and had residual pathologic disease.


 0 kommentar(er)
0 kommentar(er)
